ABOUT
Biomimetics and Biomineralization Research
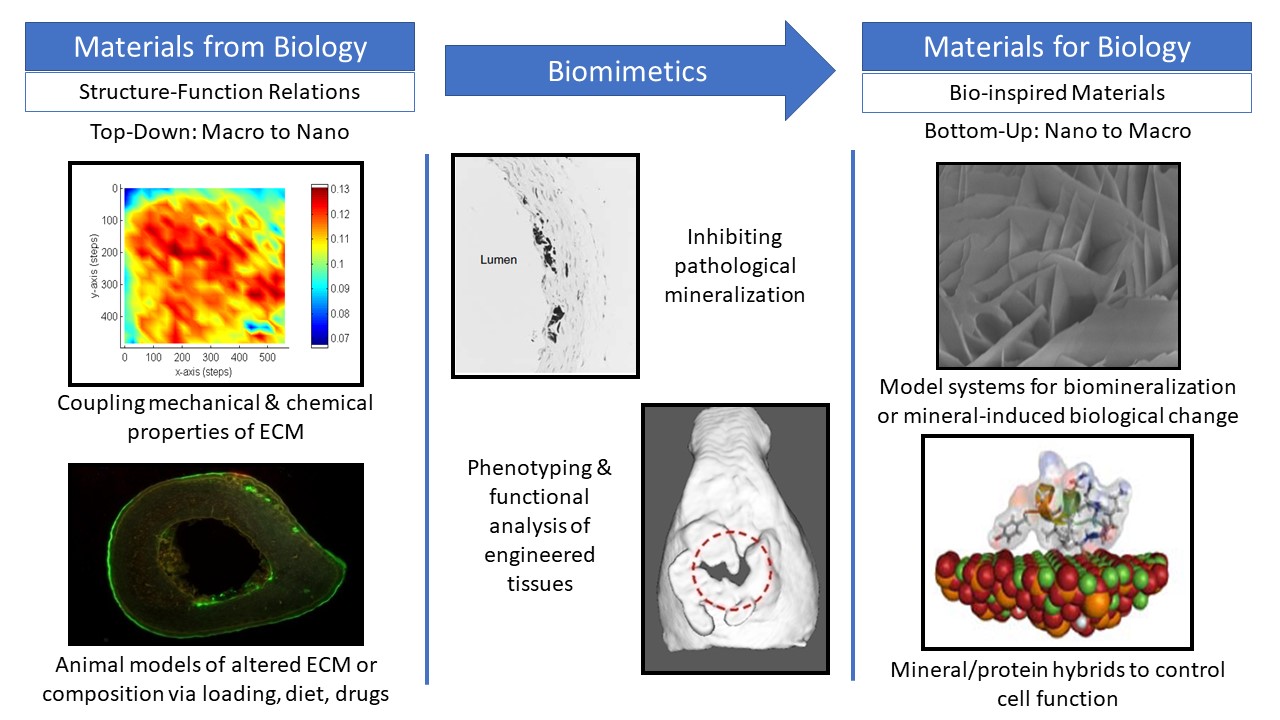
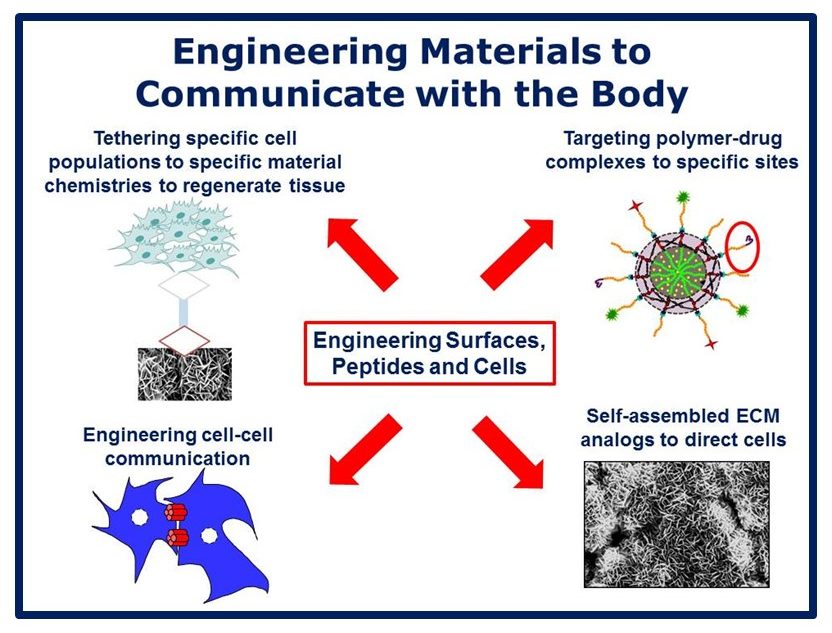
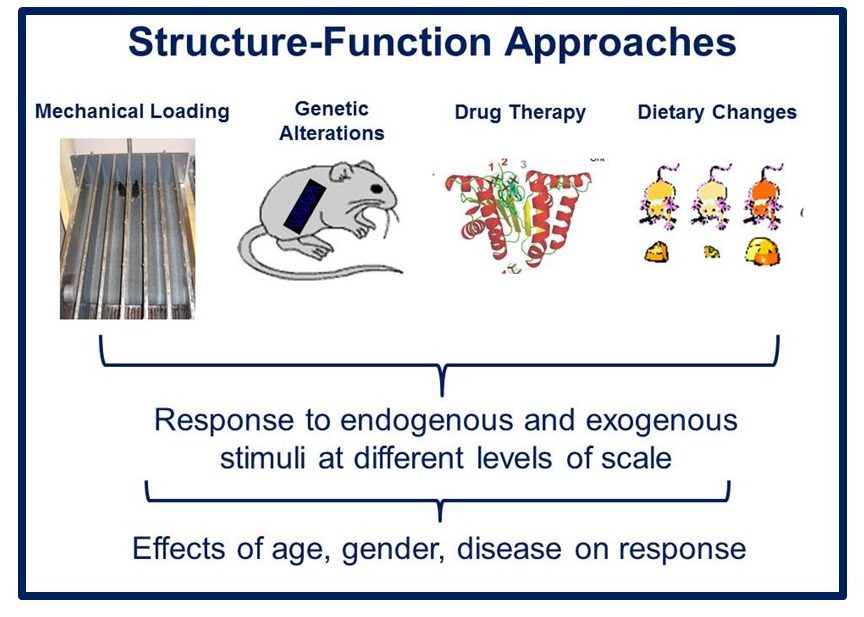
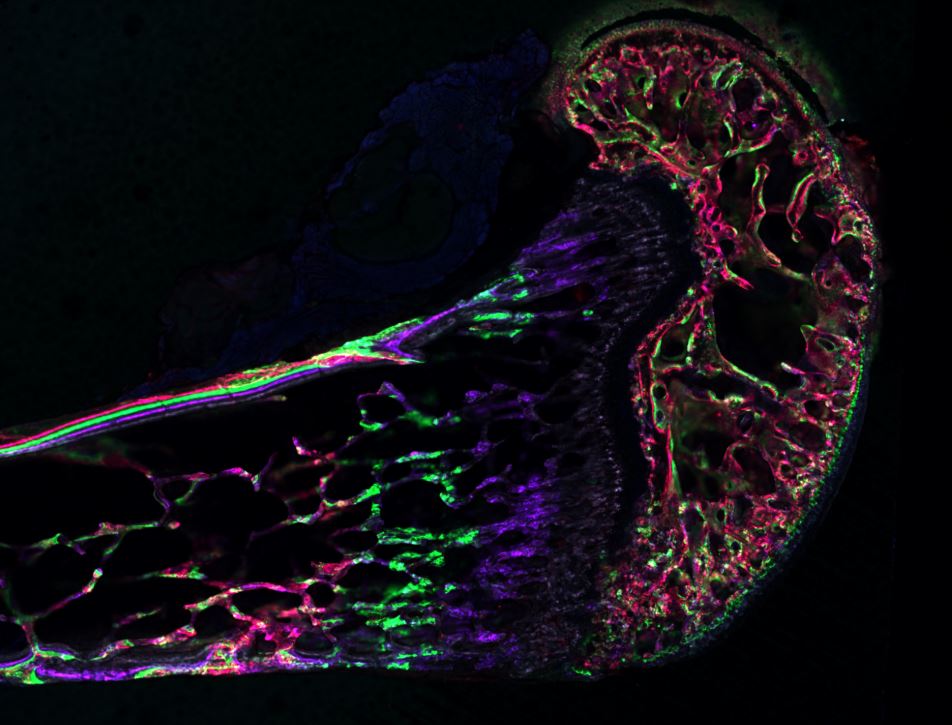
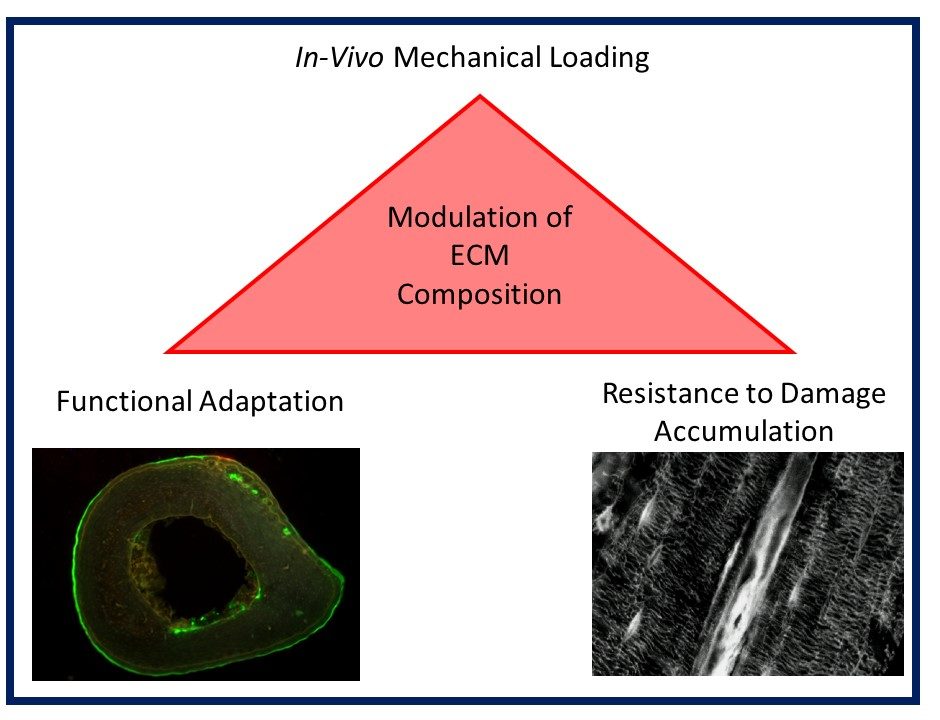
Musculoskeletal disorders incur more health care costs than breast, lung and prostate cancer combined and consume 6% of the US gross domestic product (US Bone & Joint Initiative: The Burden of Musculoskeletal Diseases in the US, 2018). Osteoporotic fractures are responsible for more hospitalizations in the US than heart attacks, strokes, and breast cancer combined (National Osteoporosis Foundation, 2019). Our laboratory is dedicated to understanding causes of skeletal fragility and developing technologies to regenerate skeletal tissue. We investigate mineralized tissues across many levels of scale to establish structure-function relations across their hierarchy and utilize this information to develop biomimetic strategies to regenerate tissues. By coupling mechanical, compositional and molecular analyses of tissues, we investigate mechanisms of skeletal fragility and mechanically mediated tissue adaptation. Using principles of biomineralization, we design materials that can communicate with their biological microenvironment, leading to better control of cell function in-vitro and tissue formation in-vivo. Our work has been funded from the NIH, NSF, DoD, and industry. We have published over 145 peer-reviewed papers and book chapters, hold 10 patents, and have given over 130 invited presentations. Dr. Kohn has mentored 45 graduate students, 7 post docs, 50 undergraduates, 14 residents and 5 visiting professors in his laboratory and many past trainees are now funded independent investigators.