Biomaterials & Biofabrication
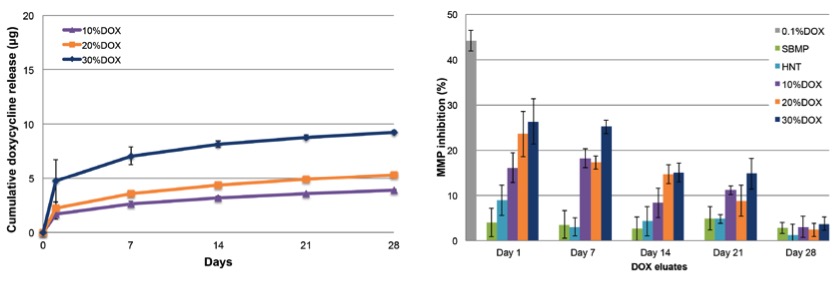
We have previously pioneered the development of a multilayered and tissue-specific membrane via electrospinning. The innovative membrane was designed and processed to present a core layer (CL) and 2 functional surface layers (SLs) that interface with hard and soft tissues. These SLs are highly customizable and can be used as a platform for generating a wide range of membranes/scaffolds endowed with unique therapeutic properties. A series of publications reflect our efforts in the fabrication of these biomaterial scaffolds for periodontal tissue engineering. We have now expanded the focus in this area with the acquisition of a unique 3D Bioprinting platform (3DDiscoveryTM, RegenHU, Villaz-St-Pierre, Switzerland) to engineer bioactive patient-specific membranes/scaffolds to amplify hard and soft tissue regeneration.
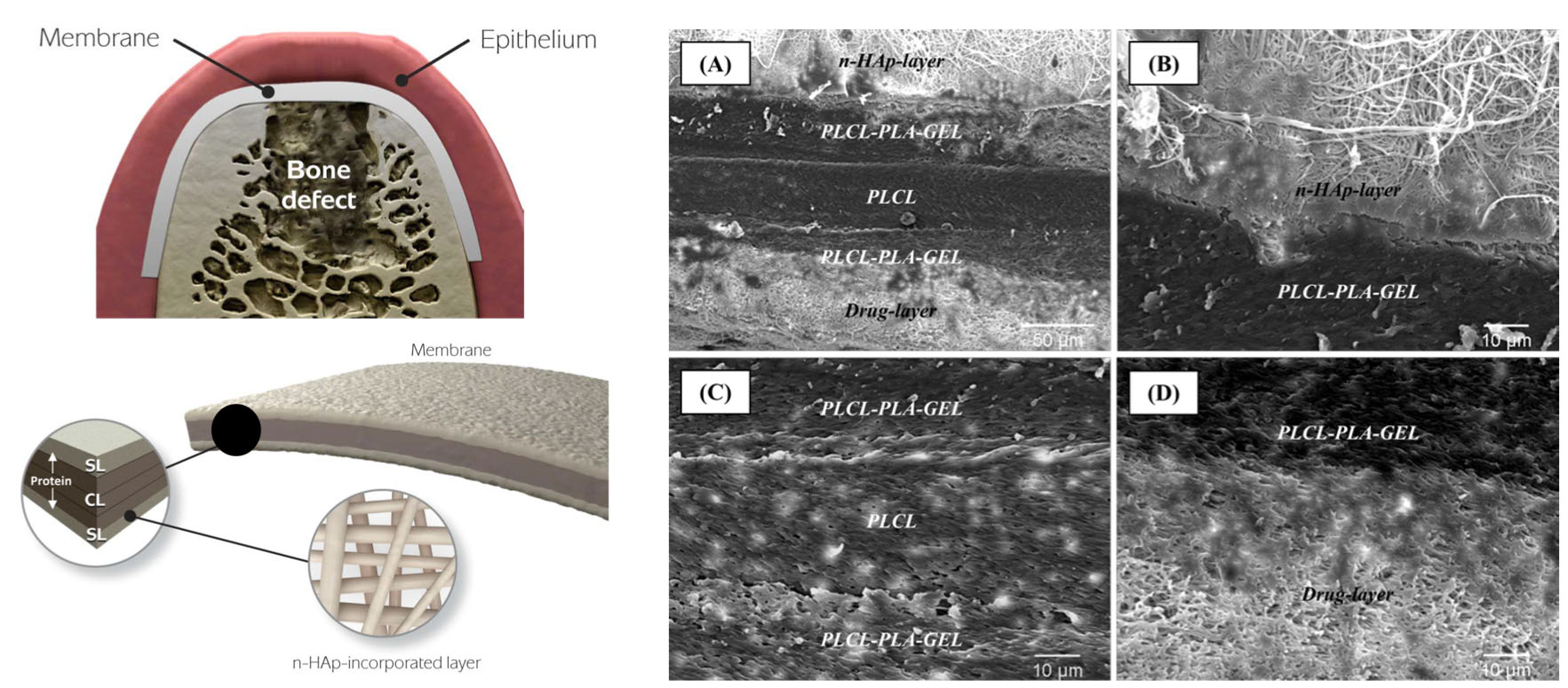
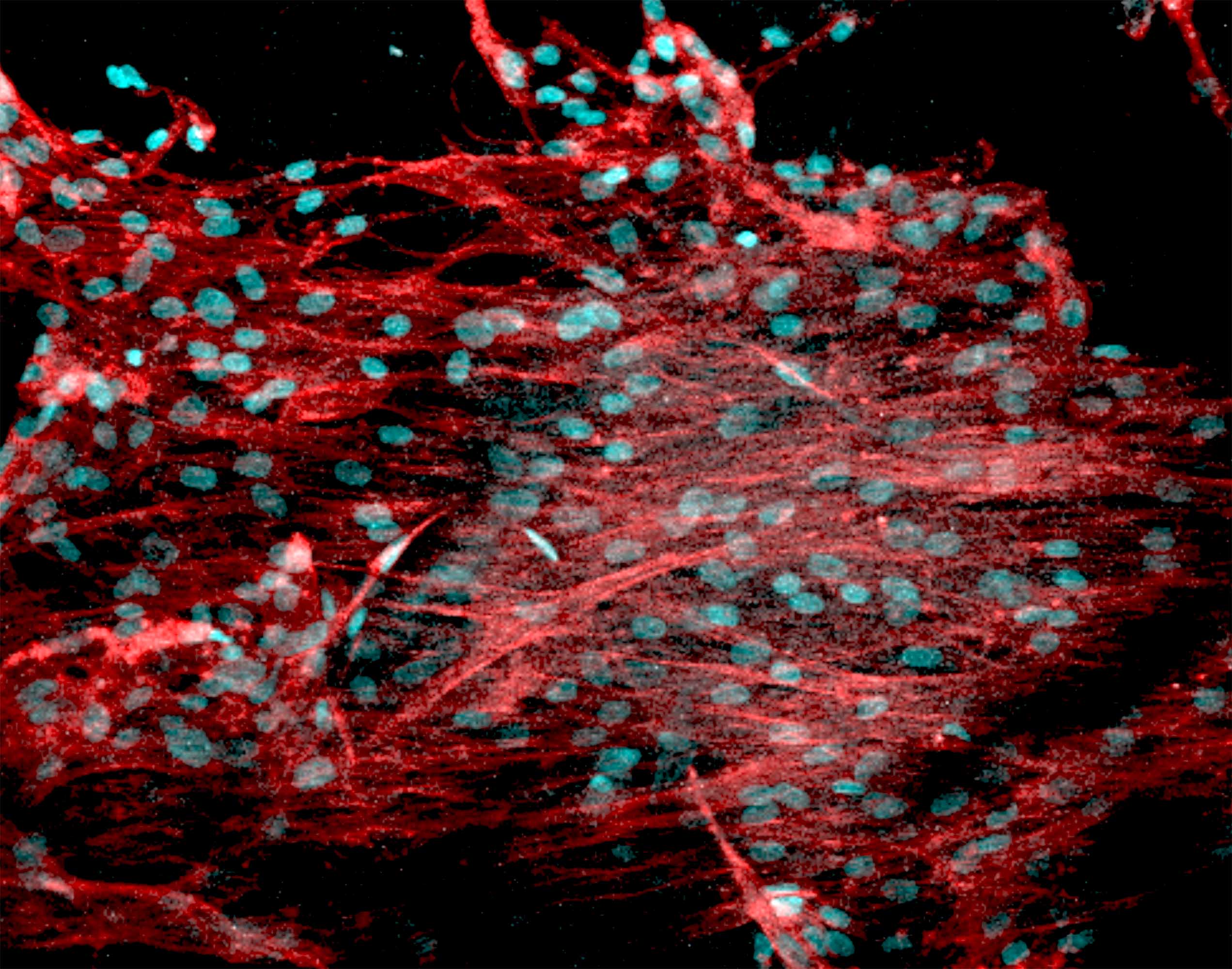
Multilayered and tissue-specific membranes/scaffolds for periodontal regeneration. (Left) Multilayered periodontal membrane processed via electrospinning showing the details of the core layer (CL) and the functional surface layers (SL). (Right) Cross-sectional SEM micrographs of the multilayered membrane.
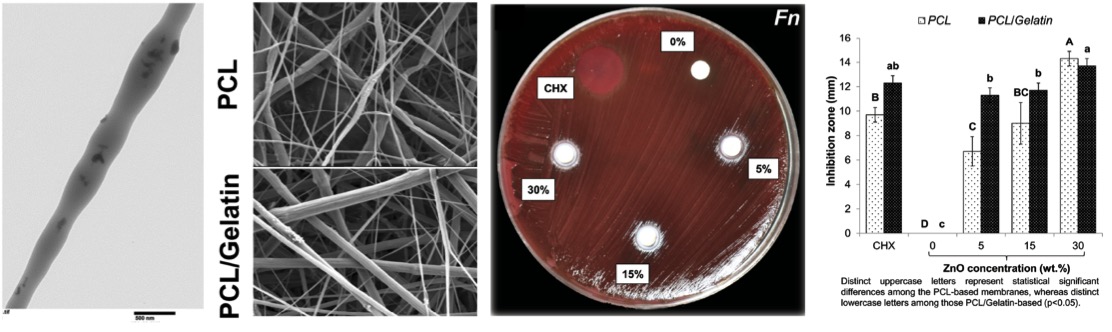
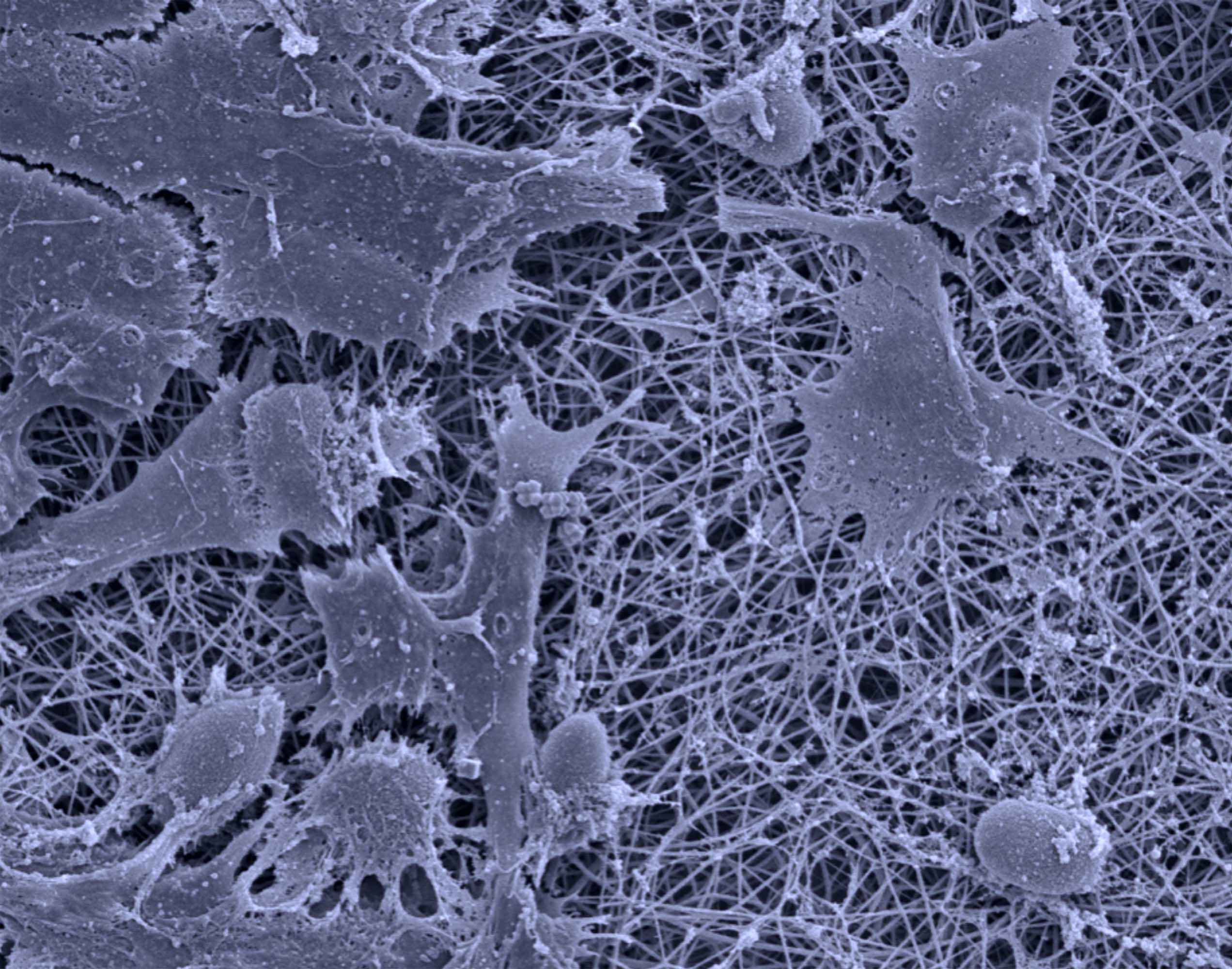
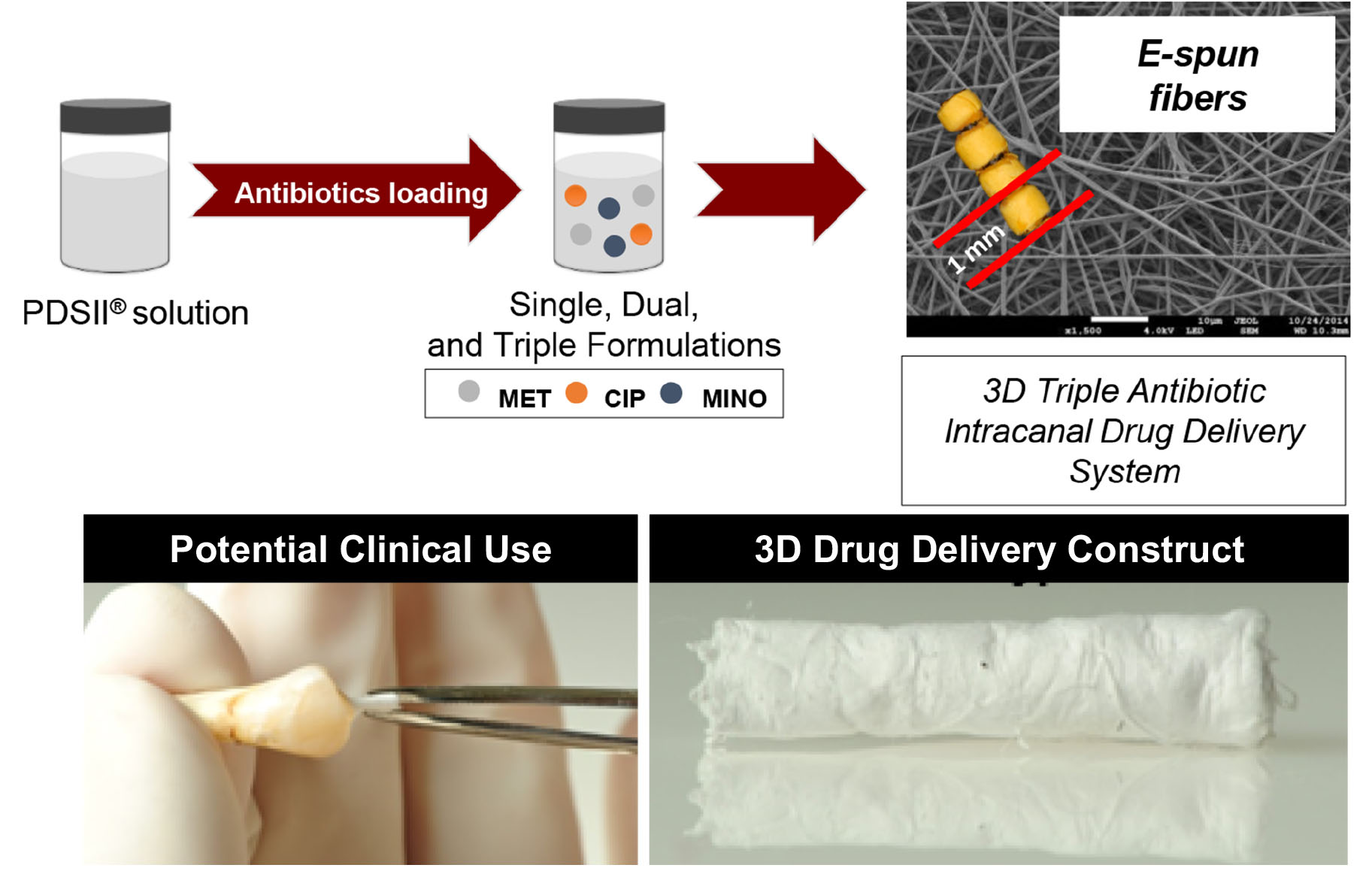
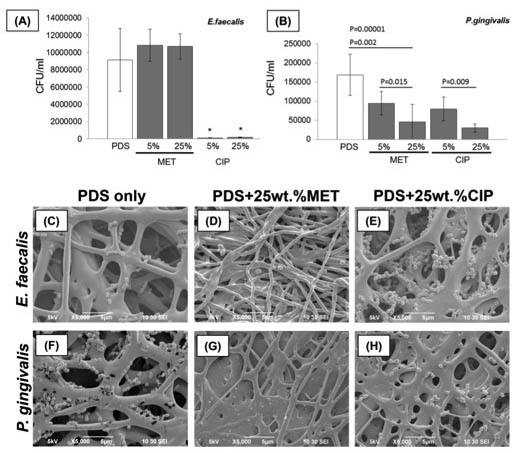
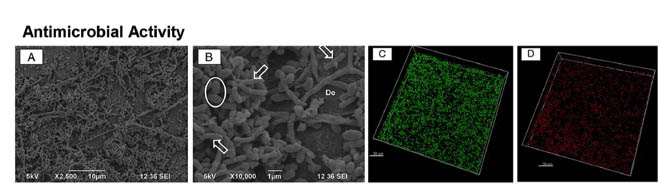
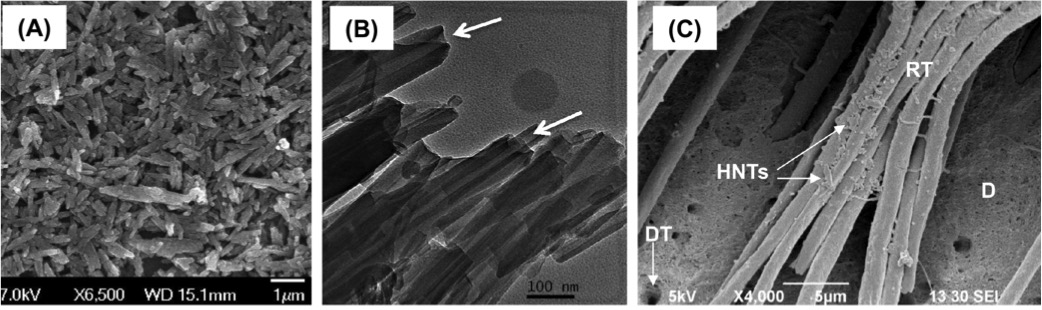
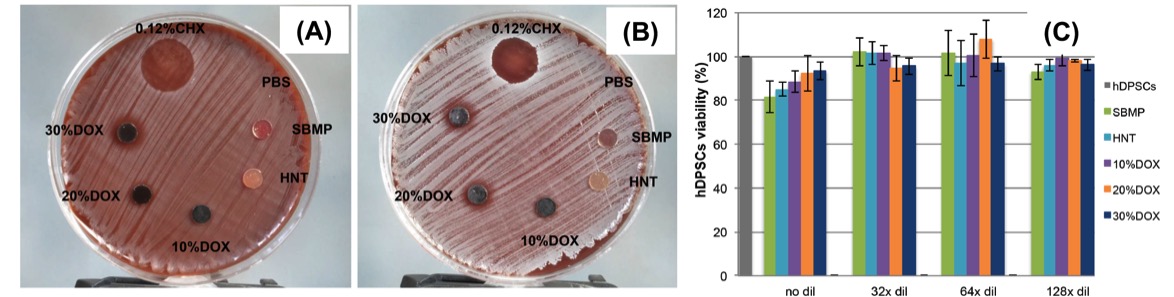
Antimicrobial properties of ZnO-decorated polymeric nanofibers. Transmission electron micrograph of a single PCL nanofiber incorporated with ZnO nanoparticles. Representative agar plate and data of the antibacterial activity of PCL and PCL/gelatin-based ZnO-decorated membranes against Fusobacterium nucleatum (Fn).

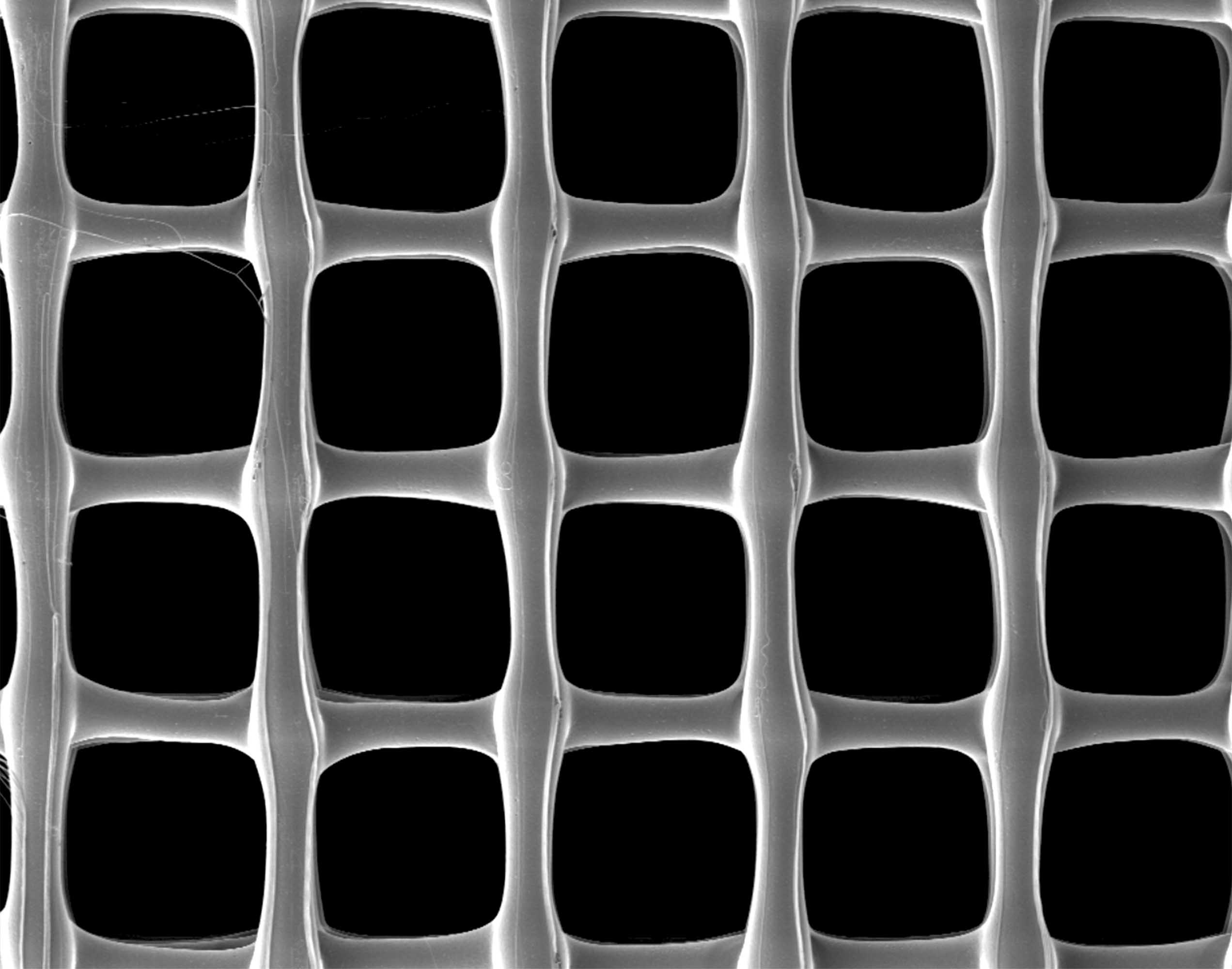
Three-dimensional bioprinting for applications in dental, oral, and craniofacial (DOC) tissue regeneration. 3D Bioprinting & Melt Electrospinning Writing (MESW) platform (3DDiscoveryTM, RegenHU, Villaz-St-Pierre, Switzerland). Scanning electron microscopy image of a 3D printed scaffold.